Anti-Wolbachia Consortium (A·WOL): Developing new drugs against onchocerciasis (river blindness) and lymphatic filariasis (elephantiasis)
2007 – present

The challenge
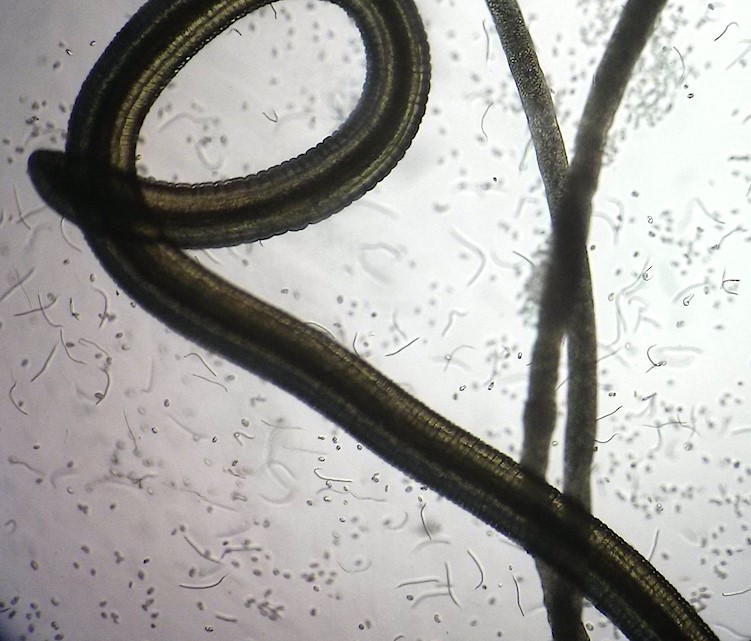
Onchocerciasis (river blindness) and lymphatic filariasis (elephantiasis) are debilitating neglected tropical diseases (NTDs) affecting over 150 million people, with more than 1.5 billion at risk of infection, primarily in low-resource settings in more than 80 countries.
Current treatments rely heavily on mass drug administration (MDA) with drugs that must be given repeatedly over many years, and which are not always effective against all life stages of the parasites causing the diseases. These filarial nematode worms are also developing drug resistance, posing major constraints to MDA programmes.
New, fast-acting, and safe treatments are urgently needed, especially in areas where current approaches are not feasible or sufficient.
About the project
The Anti-Wolbachia Consortium (A·WOL), led by LSTM, is pioneering a novel approach to treating filarial diseases by targeting Wolbachia, an essential bacterial symbiont within the worms that cause onchocerciasis and mosquitoes that cause lymphatic filariasis.
By eliminating Wolbachia, A·WOL’s therapies disable the parasites’ ability to reproduce and survive, offering a safer and potentially curative alternative to existing drugs.
Our strategies & approaches
Targeting Wolbachia bacteria to stop transmission
We focus on eliminating Wolbachia, an essential bacteria that filarial nematode worms need to survive. Part of our research is understanding the processes behind the symbiotic relationship of these worms and Wolbachia. Slowing down the worms’ reproduction makes it easier and faster to treat onchocerciasis and lymphatic filariasis.
Learn more about the Wolbachia endosymbiont
Developing next-generation treatments
Current treatments only kill the larval stage of the parasite, requiring many rounds of MDA. We are focused on developing drugs that target the adult worms. Our team also identifies, tests, and refines novel compounds that can achieve high efficacy with shorter treatment regimens, ideally in a single dose.
Driving research from lab to field
We lead collaborative research and development (R&D) across discovery, preclinical, and early clinical phases to ensure that new treatments are safe, effective, and suitable for real-world use.
Our key findings & impact
Established a new treatment pathway
We validated a breakthrough approach to treatment by proving that targeting Wolbachia bacteria can safely and effectively disable adult filarial worms. This work laid the foundation for an entirely new class of macrofilaricidal drugs, which could potentially reduce treatment timelines from years to weeks.
Advanced promising drug candidates
We advanced multiple drug candidates through early-stage clinical development, including Tylosin Analogue Macrofilaricides (TylAMac™), which has shown promising results in shortening treatment duration (≤7-day regimens) and improving patient outcomes. These compounds offer safer alternatives in regions where current drugs, such as doxycycline, might pose risks.
Expanded treatment access for communities
We support the shift from control to elimination through clinical trials and ethical, community-based research, especially in hard-to-reach populations excluded from traditional MDA campaigns.
Contributed to global elimination goals
By developing new tools aligned with WHO’s 2021-2030 Roadmap, and through partnerships with researchers, implementers, and policymakers, including in endemic countries such as Cameroon and the Democratic Republic of Congo, we help countries take the next steps toward eliminating onchocerciasis and lymphatic filariasis for good.