Caffeine citrate for Preterm infants in Africa project (CaPIA)
Caffeine citrate for Preterm infants in Africa project (CaPIA)
2023 – present
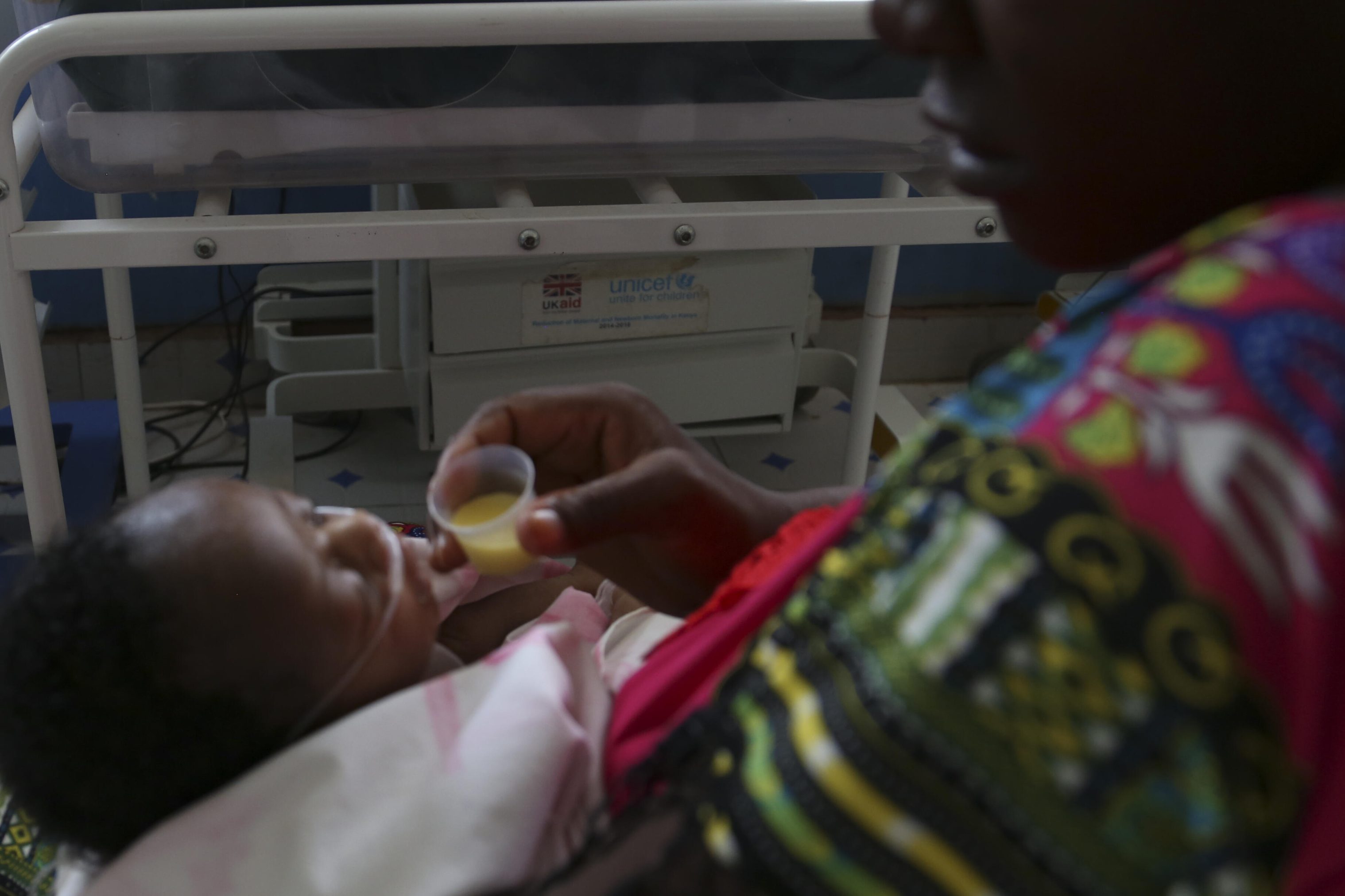
A lack of data
Apnoea of prematurity (AOP) among preterm infants in Africa is a leading cause of severe illness and death, especially in absence of regular monitoring and breathing support. In high income countries caffeine citrate is an established treatment for AOP in among preterm infants. However, there is limited data from low- and middle-income countries on its usefulness among preterm infants. There is a critical need to study the impact of caffeine citrate on important clinical outcomes in premature infants in LMICs. However, because of the lack of clinical equipoise the exact study design to achieve this goal, especially the use of placebo is complex and debatable.
Our aim is to gain consensus on the optimal study design(s) to test the impact of caffeine citrate on critical clinical outcomes in this setting.
We are conducting an adapted online Delphi survey, among >70 local stakeholders in newborn care across >20 countries in Africa (clinicians and allied professionals), to gain consensus on the optimal study design to test the impact of caffeine citrate on critical clinical outcomes in this setting. We will use our findings to support stakeholders with the decisions on how to generate evidence to inform policy strategies on caffeine citrate use in the management of apnoea of prematurity in Africa.
Publications
Nabwera HM, Ekhaguere OA, Kirpalani H, Burgoine K, Ezeaka CV, Otieno W, Allen SJ, Embleton ND; Neonatal Nutrition Network (NeoNuNet). Caffeine for the care of preterm infants in sub-Saharan Africa: a missed opportunity? BMJ Glob Health. 2021 Dec;6(12):e007682. doi: 10.1136/bmjgh-2021-007682.